Effect of Impurities and Temperature on Surface Tension of Liquid
Effect of Impurities and Temperature on Surface Tension of Liquid: Overview
This Topic covers sub-topics such as Factors Affecting Surface Tension, Effect of Contamination on Surface Tension, Impurity Effects on Surface Tension and, Temperature Effects on Surface Tension
Important Questions on Effect of Impurities and Temperature on Surface Tension of Liquid
At what temperature, will the surface tension of water, be minimum ?
What are the factors that affect the surface tension of a liquid?
If there is dust, grease, oil etc. on the liquid surface, then the surface tension _____.
Ambient contamination produced a sharp decrease of the surface tension of ultra-pure water.
How does temperature affect the surface tension?
What is the effect of contamination and temperature on the surface tension of a liquid?
What kind of impurities increases surface tension?
Surface Tension of a liquid is due to
How does soap decrease the surface tension of water. Explain in brief.
Impurities always decrease the surface tension of a liquid.
When a sparingly soluble substance like alcohol is dissolved in water, surface tension of water
The surface tension of water at is . Find surface tension of water at ( for water
Explain the effect of presence of impurities on the surface tension of liquid.
Given below are two statements:
Statement I : If a capillary tube is immersed first in cold water and then in hot water, the height of capillary rise will be smaller in hot water.
Statement II : If a capillary tube is immersed first in cold water and then in hot water, the height of capillary rise will be smaller in cold water.
In the light of the above statements, choose the most appropriate from the options given below
Given below are two statements :
Statement (I) : Viscosity of gases is greater than that of liquids.
Statement (II) : Surface tension of a liquid decreases due to the presence of insoluble impurities.
In the light of the above statements, choose the most appropriate answer from the options given below :
Which of the following is compound?
The surface tension and vapour pressure of water at is and respectively. The radius of the smallest spherical water droplet which can form without evaporating at is
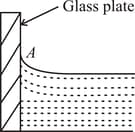
A glass plate is kept inside water (angle of contact ) as shown in the figure. The shape of curvature of free surface of water at point A is
A water-proofing agent changes the angle of contact
At which of the following temperatures, the value of surface tension of water is minimum ?
